19 May 2022
In their 2022 retailer index report, The Access to Nutrition Initiative calls UK retailers’ commitment to infant and young child nutrition ‘sparse’ and urges adherence to the Code
The Access to Nutrition Initiative’s (ATNI) 2022 UK retailer index marks the first full nutrition- and health-specific index to assess all major food retailers within the UK market and their contribution to healthy, affordable diets. The index is based on eight nutrition-related topics and is the latest in a suite of materials produced by ATNI following publication of the UK Supermarket Spotlight 2020 and two UK Product Profiles (2019 and 2021).
Key findings
Infant and young child nutrition scored the lowest of all topic areas examined in the report with an average of just 1.1 points out of 10. The index described UK retailers’ commitment to infant and young child nutrition as ‘sparse’.
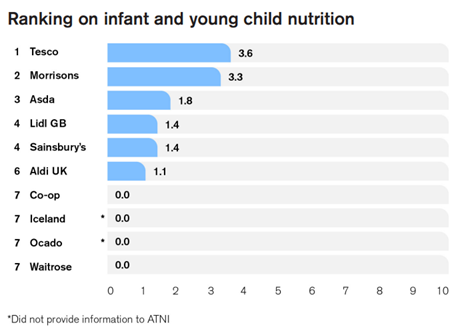
The table above shares how each retailer ranked on infant and young child nutrition (p108).
Adherence to the Code
Zero out of eight retailers were found to make commitments to the International Code of Marketing of Breastmilk Substitutes and subsequent World Health Assembly (WHA) resolutions (the Code) in their marketing of breastmilk substitute (BMS) products. Any actions which were identified followed only UK standards, falling short of World Health Organization (WHO) recommendations. Three retailers did state their compliance with UK regulations on infant and follow-on formula (p13).
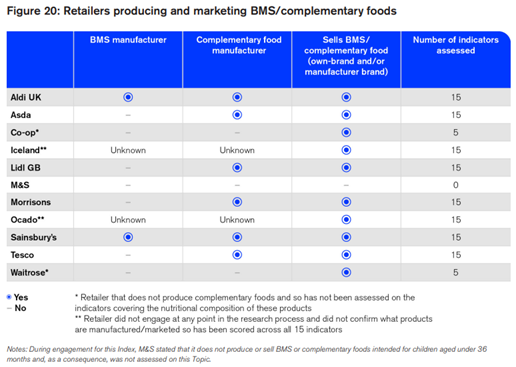
The table above depicts the retailers’ status as a BMS manufacturer or seller (p104).
None of the 10 retailers that sell BMS explicitly commit to marketing these products in compliance with the Code and no specific mention was found of the Code during research for the index, suggesting that the retailers are not familiar with it.
Breaches of the Code were found during the research, with promotions for BMS products aimed at children over six months and for complementary foods aimed at children from four months. (p109)
Actions required
The index sets out key recommendations for retailers who sell BMS or complementary food and/or who manufacture own-brand complementary food. These include:
- adhering to the Code and public disclosure of such adherence
- adhering to existing national, regional and international guidance on the nutritional composition, marketing and labelling of complementary foods
- ensuring that the nutritional composition of own-brand complementary food products aligns with the nutrient profiling standards set out in WHO/Europe’s proposed guidance
- ensuring that labelling requirements and promotional criteria of complementary foods align with the provisions set out by WHO.
See p111 for full list of recommendations.
There are significant concerns about breastmilk substitutes (BMS) and commercially produced complementary (solid) foods. The inappropriate marketing of BMS has been shown to be a contributing factor in decreased rates of breastfeeding, which highlights the need for governments, manufacturers, retailers and distributors to adopt and implement regulations and standards that are fully aligned with WHO recommendations – recommendations that are not currently fully implemented in UK legislation.
To read the full report, visit The Access to Nutrition Initiative’s website here.
Learn about the Code and UK law
The UK Law
The UK regulates the marketing of breastmilk substitutes through the Infant Formula and Follow-on Formula Regulations 2007 and accompanying guidance notes which are designed to help with interpretation of the law. The regulations implement the European Commission Directive 2006/141/EC which is intended to ‘give effect to the principles and aims of the WHO Code’. The UK regulations are intended to ‘regulate labelling and restrict advertising and presentation of infant and follow-on formula so as not to discourage breastfeeding’. However, they are not as robust as the Code and so the companies find ways around the law. One of the biggest weaknesses is that, while the Code considers follow on formula (i.e. milk marketed for babies over six months) as a breastmilk substitute, the UK law does not. This allows the companies to advertise their brand name and logos on TV, online in magazines and elsewhere.
Baby Friendly in the UK
Services working towards Baby Friendly accreditation are required to adhere to the International Code of Marketing of Breastmilk Substitutes (the Code) and must ensure that there is no advertising of infant formula, bottles, teats or solid food for babies under six months old to mothers and their families. This requirement is intended to restrict the influence of commercial interests related to infant feeding. It does not in any way prohibit the provision of factual information about bottle feeding or introducing solid food or require that mothers who bottle feed be denied information or care. It is intended to ensure that all parents, whichever way they feed their baby, have access to accurate and effective information free from the influence of marketing campaigns designed to protect profits rather than babies.
We work with health professionals to support all parents who formula feed by providing information on making up feeds and feeding responsively and as safely as possible and how feeding can be a source of not only nutrition, but also of love, comfort and reassurance between babies and parents. Crucially, we support health professionals to provide compassionate, non-judgemental and mother-centred support.
- Learn more about the Code in the UNICEF UK Baby Friendly Initiative Guide for Health Workers and our selection of resources on the Baby Friendly website.
- For research on infant nutrition, please visit the Baby Friendly research pages.